Stability of
Emulsion :
A stable emulsion may be defined as one in which the
disperse phase retain their initial character and remain uniformly distributed
throughout the continues phase.
Stability may be defined in term of physical and chemical stability.
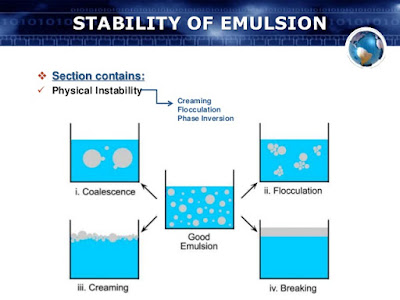
Physical Stability: Physical stability is that type of stability of emulsions
which is associated with the physical characters of emulsions. It may be due to
creaming, aggregation or flocculation, coalescence and inversion.
Creaming and Sedimentation : Creaming is due to the upward
movement of disperse phase. Creaming leads to coalescence and breakage of
emulsion. Creaming occur when the density of disperse phase is less then the
continues phase. Creaming is reversible phenomenon and can be easily reversed
by mechanical agitation for a shorter time.
Disadvantages of Creaming:
1) it leads to inelegant emulsion.
2) Dose disturbances may
occur.
3) creaming may cause coalescence and further breakage of emulsion.
Remedy: Stocks
law define remedies both for creaming and sedimentation. Stokes law
V2=d2∆Ꝿ g/18η
D2 is the diameter of the globules reducing the
diameter of the globules V (velocity) of creaming will decrease and thus lesser
chance for creaming. Small particle size of disperse phase lesser the chances
of creaming.
∆Ꝿ is the difference between the density of
disperse phase and disperse medium. Higher the difference the greater will be
the chance of creaming.
g is the gravitational velocity.
η is viscosity------- increasing the viscosity
creaming chances will be reduced. Viscosity can be increased by adding
viscosity builders like Methyl cellulose in O/W emulsion and addition of soft
paraffin in W/O emulsion. Storing the emulsion at low temperature (above the
freezing point ) increase the viscosity of emulsion thus lower the creaming
phenomenon.
Proper ratio
of Disperse phase to Disperse medium: One
approach to reduce creaming is to have proper ratio of Disperse phase to
disperse medium. The ratio of less then 20 % will readily lead to creaming. It
is theoretically possible to have disperse phase ratio up to 74 % but in
practically it is not more then 60% and some studies prefer 50% not more.
Flocculation:
Flocculation is the aggregation of globules
into a loose cluster. The globules retain their individual integrity but are
just in aggregated form. Flocculation enhance the chance of creaming and it is
the preceding step in coalescence. Flocculation is a reversible phenomenon and
can be disturbed by agitation.
Flocculation is an electrical phenomenon in which the globules stick
together due to some sort of attraction between them.
Remedy : By adding possible effective surfactants
possessing a suitable electric charge to prevent this phenomenon.
Coalescence:
Coalescence is the growth process in which
small particle/globules of disperse phase merge together and form a large
particle, which further merge and lead to separation of two phase (breaking).
As said causes of coalescence are creaming and flocculation. Both of these
precede the coalescence. Coalescence is the irreversible phenomenon. It is
differ from flocculation in irreversibility and integrity of particle (in
flocculation no merging occur) and another difference between is that
coalescence dose not depends on charge.
Reasons: coalescence occur when there is the breakage
of interfacial film of the emulsifiers around the dispersed globules.
Remedy: Coalescence can be overcome by adding Mix
emulsifying agents which give
1)Elastic and rigid film.
2) Viscous film (Elastic and viscous film reduces
the chances of Coalescence
3) cautious use of HLB scale for selection of
emulsifying agents.
Inversion
: Inversion is also one of the problem
with the emulsion. Inversion is the process in which o/w water emulsion turns
to w/o emulsion or vice versa. This phenomenon is due to
Temperature: high temperature may change the
characteristic of emulsifying agent thus promote phase inversion i.e polysorbates stabilized o/w emulsion is
inverted to w/o because of the breakage of H bonds in polysorbates due to
heat. Temperature at which inversion
occur is called phase inversion temperature (PIT).
Electrolyte addition: Sodium-oleate which
stabilize O/W emulsion when Calcium chloride is added to it turns to W/O
emulsion because calcium oleate is more soluble in oil.
Ratio of disperse phase to disperse medium:
using a high ration above 60 percent of dispersed phase inverses the emulsion.
Limit of disperse phase is 74 % which is
impractical.







Thanks for your superb post and selected article on your blog, We wishes that you offer some time with us.
ReplyDeleteYou are Welcome. That will be my pleasure
Deletegot lot of information by ths blog
ReplyDeleteprescription discount card online
Welcome
DeleteThis is My Pleasure Jhon Mac. You are Welcome
ReplyDeleteThanks for the detailed explanation!
ReplyDeleteThis really helps for my Pharmacist licence exam!
You are welcome dear
DeleteTq
ReplyDeleteGood explanation of the question
ReplyDelete